About Event
About the 9th Liquid Biopsy for Precision Oncology Summit
2025’s 9th Liquid Biopsy for Precision Oncology Summit returned bigger and better than ever before, with an expanded focus on emerging technologies in ctDNA and MRD testing, and brand-new sessions on bioinformatics, technology innovation, novel analytes, underserved therapeutic areas, regulatory updates, and much more.
The refreshed speaker faculty was peppered with new voices from pioneering biotech, including Repare Therapeutics, Affini-T Therapeutics, Vividion Therapeutics, Bantam Pharmaceuticals, and IDEAYA Biosciences, bringing new perspectives on overcoming critical challenges, from integration into clinical trials, to managing commercialization hurdles.
Our attendees enjoyed enhanced networking opportunities that offered more chances to connect with peers and industry leaders, making this the most comprehensive conference for liquid biopsy professionals to date.

ATTENDEES FROM PRECISION ONCOLOGY

WORLD-CLASS SPEAKERS

BIOTECHS NEW TO THIS MEETING

HOURS OF
IN-PERSON NETWORKING

PRE-CONFERENCE WORKSHOP & ENGAGER SESSIONS

DEDICATED TRACKS OF CONTENT
What You Missed:

Pioneering MRD
as a clinical endpoint, tackling clinical validity hurdles, to unlock non-invasive testing and
more efficacious therapies for patients with unmet needs



Leveraging
whole-genome and single cell technologies to boost the speed, sensitivity, and specificity of
your diagnostics, enabling faster, more accurate diagnoses for personalized treatment options



Optimizing your
clinical trial design, using liquid biopsies to transform your patient selection strategies
and refine your dosage regimes to enhance patient response to precision therapies


Harnessing
untapped analytes, such as RNA and exosomes, to unlock critical insights in drug-diagnostic
development that could enhance treatment outcomes



Streamlining
reimbursement pathways to drive adoption of liquid biopsies, maximizing the commercial
success of your drug-diagnostic and more accessible for patients in need



Overcoming
regulatory hurdles such as the Final LDT Rule, IVDR, and other global challenges, to
fast-tracking your therapeutic market entry and maximize the impact of your drug for patients


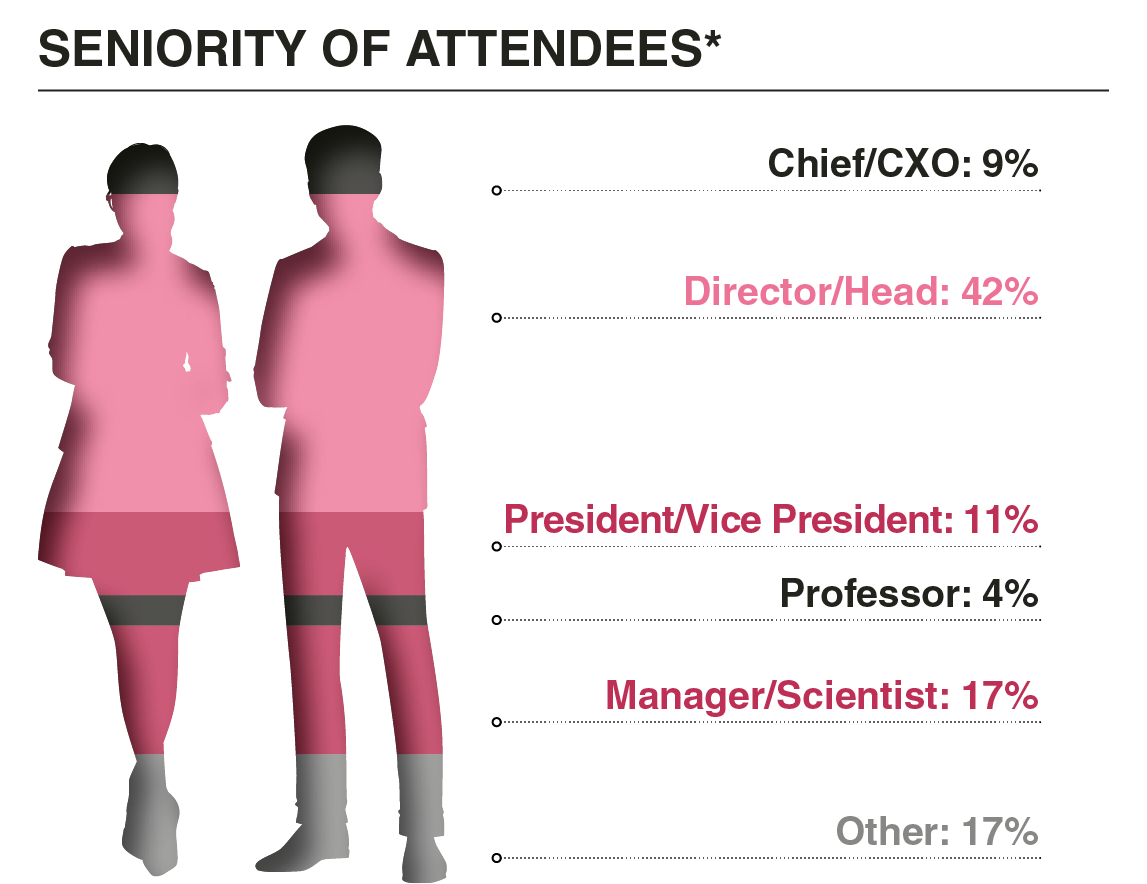
Who Did You Meet?
This meeting united both new and established leaders from companies pioneering liquid biopsy. Our goal was to spark meaningful conversations and collaborations that drive innovation in precision oncology.
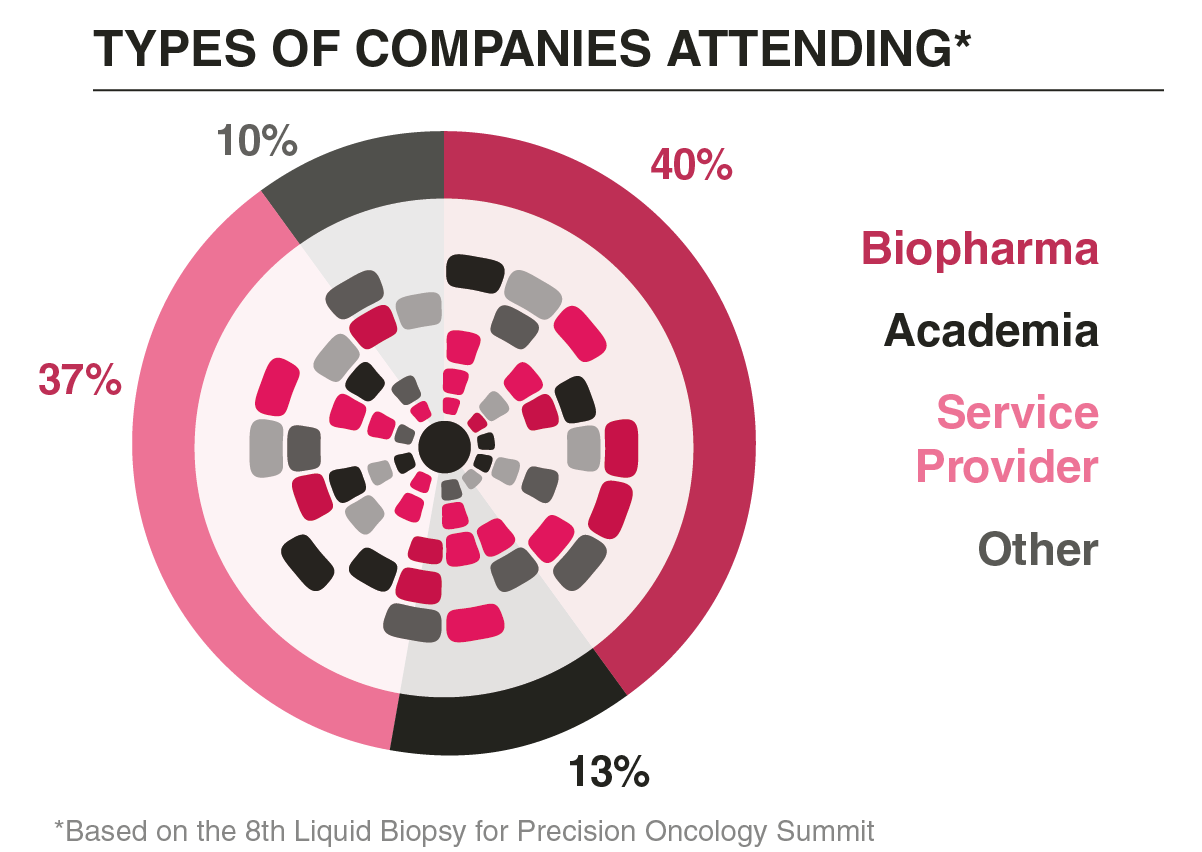
Previously Attending Companies Include:
What Your Peers Have To Say:
“Extremely informative and great networking opportunities.”
Past Attendee, Associate Director - Computational Chemistry, 858 Therapeutics
“Outstanding scientific presentations with very engaging speakers”
Past Attendee, Executive Director - Medical & Head of Oncology, Fortrea
“Well organized; speed networking segment resulted in two meetings that could lead to potential partnerships”
Past Event Partner, Vice President of Biopharma Services, Sophia Genetics







